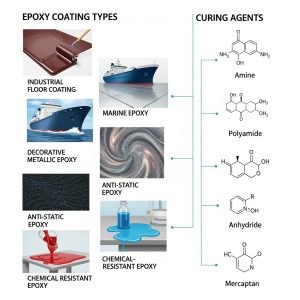
Introduction
Curing agents play a pivotal role in the performance and durability of modern coating systems. As the demand for specialized coatings increases across industries from automotive and electronics to infrastructure and marine,understanding the behavior of curing agents becomes essential. These agents are responsible for initiating crosslinking reactions with polymers, transforming liquid formulations into robust, chemically resistant films.
This article explores the types, characteristics, and application-specific performance of different curing agents Whether you are developing solvent-borne, waterborne, or specialty coatings, selecting the right curing agent is critical to achieving long-term performance and processing efficiency.
Let’s get started.
What are Curing Agents?
In modern coating formulations, the final film typically forms through a crosslinking reaction. This reaction occurs when the base polymer interacts with a curing (or crosslinking) agent to create a solid network.
A curing agent is any substance that actively reacts with oligomers, pre-polymers, or polymers to drive polymerization and complete film formation. Because it is consumed in the reaction, the curing agent becomes an integral part of the finished film, directly influencing its mechanical strength and chemical resistance. Working alongside the binder, it helps establish a three-dimensional network, a process that can be further tuned with a suitable catalyst.
Since the curing agent participates in polymerization, coatings produced with it exhibit mechanical and chemical properties that differ markedly from films formed without one. The accompanying table and figure illustrate how polymerization pathways and resulting film characteristics change when a curing agent is present versus absent.
Since the curing agent actively takes part in the polymerization process, the mechanical and chemical characteristics of the coating system vary depending on whether a curing agent or crosslinker is used. The equation below illustrate the differences between polymerization with and without a curing agent:
Reaction Comparison:
- Without Curing Agent: A + A → A-A-A
- With Curing Agent: A + B → ABA-ABA
- In some cases, polymerization cannot begin without the presence of a curing agent.
- The curing agent reacts with the polymer components, becomes part of the final film structure, and contributes specific properties by helping to form a three-dimensional network.
Polymerization With vs. Without Curing Agent
Why a Curing Agent is Needed:
A curing agent, also referred to as a crosslinker, enables the polymerization process by reacting with the functional groups of the polymer material.
- After the reaction, the curing agent remains embedded within the polymer matrix.
- Certain curing agents can enhance system performance by supporting the formation of a well-structured 3D network with the binder.
Want to know more about adhesion between coating and substrate? Check out our blog on COATING’S ADHESION & COHESION
Next, let’s take a closer look at the main types of curing agents or crosslinkers commonly used in coating formulations.
Types of Curing Agents in Coatings
There is a wide range of curing agents available, and their applications are not limited to specific coating systems. This is because many polymers share common reactive groups, such as –OH or –COOH, which can interact with different curing agents. For this reason, it is more useful to focus on the characteristics of the curing agents themselves rather than the base polymers.
Choosing the right curing agent is essential to achieving high-performance coatings. When the correct agent is selected, it enables the formation of an optimal three-dimensional network, which significantly improves the final film’s properties.
Curing agents are compatible with various coating technologies, provided their physical form matches the system requirements. They are commonly used in waterborne coatings, solvent-borne coatings, powder coatings, and radiation-cured systems. Let’s look at the different types of curing agents
Amines and Polyamines
Amine-based curing agents are commonly used in epoxy systems due to their strong reactivity with epoxy groups, which leads to the formation of a durable, crosslinked network. These agents vary in structure and performance, allowing formulators to tailor properties for specific applications. The main categories include aliphatic amines, cycloaliphatic amines, and aromatic amines, each with unique characteristics.
Aliphatic Amines
Derived from straight or branched chain aliphatic compounds, these curing agents offer good chemical resistance, flexibility, and strong adhesion. They are frequently used in coatings, adhesives, and sealants.
Cycloaliphatic Amines
These amines contain saturated ring structures, giving them excellent resistance to UV exposure and yellowing. They are ideal for outdoor and high-durability applications, such as epoxy flooring, protective coatings, and advanced composites.
Aromatic Amines
Recognized for their superior chemical resistance and mechanical strength, aromatic amines are suited for demanding environments. They are often used in structural adhesives, tank linings, and chemical-resistant coatings where durability is critical.
Curing Behavior
Amine-based curing agents are valued for their relatively fast curing rates, often outperforming alternatives like polyamides. Depending on the specific type of amine, the formulation, and environmental conditions, cure times can range from just a few minutes to several hours. Polyamines, in particular, enable rapid curing, which requires quick application and precise handling to prevent the material from thickening prematurely.
Performance Attributes
Polyamines deliver excellent toughness and flexibility, along with strong adhesion and chemical resistance. These characteristics make them ideal for high-performance applications where quick curing and strong bonding are important, such as epoxy flooring systems, sealants, and fiber-reinforced composites.
Limitations and Safety Considerations
Despite their strengths, amine curing agents come with certain drawbacks. Compared to curing agents like anhydrides, they generally offer lower thermal resistance. Additionally, amine-cured systems can be moisture-sensitive, sometimes leading to surface imperfections like blushing or compromised performance if not applied under controlled conditions. Some aromatic amines may also raise health and environmental concerns, making it essential to implement strict safety measures during storage, mixing, and application.
Anhydrides
Anhydrides are compounds formed from organic acids that usually have two acid functional groups, which are dehydrated to produce the anhydride structure. Epoxy systems that are cured using anhydrides are well known for their excellent heat resistance, strong electrical properties, and quick curing under moderate temperature conditions.
Curing Characteristics
Anhydrides enable relatively quick curing when compared to curing agents like amines. They can reach a high level of cure in a short amount of time, which makes them useful for applications that require fast processing. In addition, anhydrides typically cure at moderate temperatures, generally falling between 100 and 180°C. This moderate temperature range supports efficient processing while reducing the thermal stress applied to substrates.
Performance Properties
Anhydrides provide strong thermal resistance, which makes them ideal for use in high-temperature environments. Epoxy systems cured with anhydrides show reliable electrical insulation, making them suitable for electronics and electrical uses such as laminates, potting materials, and encapsulants. These systems also tend to display stable dimensional properties, helping them retain form and function across varying temperatures. This stability is valuable in applications that demand tight tolerances or low thermal expansion during operation.
Considerations and Limitations
Since anhydrides are dehydrated forms of carboxylic acids, they are highly sensitive to moisture. This sensitivity can lead to problems such as blushing or lower performance if moisture is present during curing. It is important to process anhydride-cured epoxy systems in dry conditions and prevent contact with humidity. Anhydrides also tend to have shorter pot life compared to several other types of curing agents. As a result, proper planning and prompt handling are essential to avoid early curing and ensure smooth application.
Lastly, certain anhydrides like phthalic anhydride may present health risks due to their potential to cause irritation or sensitization.
Want to know more about Protective Coatings? Check out our post – Protective Coatings
Phenalkamines
Phenalkamines are a specific class of epoxy curing agents that combine the properties of aliphatic amines and phenols.Phenalkamines possess distinct curing behaviour and performance features, making them ideal for a wide range of applications.
Curing Characteristics
Phenalkamines enable fast curing, even when temperatures are low. They show high reactivity with epoxy resins, supporting short cure cycles and achieving excellent cure in less time. They are effective at curing (in a few hours) in extremely cold climates where other curing agents need several days. Moreover, phenalkamines display excellent tolerance to moisture during the curing stage. They cure reliably even with the presence of moisture or humidity, making them useful in damp areas and for construction applications. Their viscosity is lower than many other curing agents, making them easier to handle during use.
Performance Properties
Phenalkamines deliver outstanding resistance to corrosion and chemicals, protecting against many solvents, corrosive agents, chemicals, and water. They are often selected for use in environments needing strong resistance, such as tank linings, marine systems, chemical coatings, and industrial floors. Phenalkamines also ensure strong adhesion on multiple surfaces, including metal, treated metal, and concrete surfaces. This feature supports consistent bonding and reliable coating function across various applications and materials. In addition, epoxy systems cured with phenalkamines show notable flexibility and durability, which is ideal in situations involving vibration, impact, or mechanical stress. They also perform reliably at low temperatures and retain their toughness and flexibility in cold conditions, making them suitable for arctic and sub-zero temperature use.
Considerations and Limitations
Phenalkamines typically offer a shorter pot life (or working time) when compared to other curing systems. As a result, careful handling and prompt application are essential to prevent early curing or material waste. Certain phenalkamines may experience discoloration or yellowing over time, especially when subjected to UV light or outdoor exposure. Although phenalkamines tolerate moisture during curing, they can be vulnerable to moisture before curing takes place. For this reason, storing and handling phenalkamine resins in dry areas is critical to avoid water uptake or material breakdown.
Polyamides/ Polyamidoamines
Polyamides are a type of epoxy curing agent derived from the reaction between dimer fatty acids and polyamines. They are composed of long-chain molecules containing both amide and amine groups along the backbone, which results in a more complex molecular structure. As a result, they offer distinctive properties and are widely utilized across various industries. Polyamidoamines are simply lower molecular weight versions of polyamides.
Curing Characteristics
Polyamide curing agents generally offer a relatively long pot life (or working time) and cure more slowly than other types of curing agents. In most environmental conditions, this curing time typically ranges from 4 to 8 hours, which is often seen as a benefit, as it provides a longer working window for the user. However, in colder climates, the extended cure time can become a drawback, sometimes stretching into days. Although accelerators can be added to reduce the curing time, in such cases it may be more practical to switch to a different curing agent.
Performance Properties
Polyamides provide good levels of flexibility, toughness, and chemical resistance. They also demonstrate excellent adhesion to a wide range of substrates, which makes them well-suited for uses that require both strong adhesion and chemical resistance, such as coatings, adhesives, and laminates.
Considerations and Limitations
Like polyamines, polyamides generally have lower thermal resistance when compared to curing agents such as anhydrides. Additionally, epoxy systems cured with polyamides can be moisture-sensitive, which might cause surface issues like blushing or a drop in performance if not handled under controlled conditions. Moreover, polyamides typically have higher viscosities, which can make them more difficult to process and apply. Therefore, formulation adjustments or changes in the application method might be needed.

Phenalkamides
Phenalkamides represent a newer category of epoxy curing agents that combine the features of phenalkamines and polyamides. Phenalkamide curing agents are specifically designed for reliable performance in all-weather conditions. They are also highly suitable for tropical regions, where polyamide hardeners are typically chosen instead of phenalkamines due to their shorter pot life.
Curing Characteristics
Phenalkamides offer a practical pot life that closely matches polyamide resins under tropical climates. They usually possess viscosity that is less than polyamides but greater than phenalkamines and react more quickly with epoxy resins in comparison to polyamide-based systems. Also, they are capable of curing efficiently in colder temperature environments.
Additionally, similar to phenalkamines, phenalkamides show reliable moisture resistance during curing, allowing use in damp or humid areas. Their viscosity tends to be lower than polyamides while remaining above that of phenalkamines.
Performance Properties
Phenalkamides, much like phenalkamines, offer strong resistance to corrosion and chemicals, protecting against many solvents, chemicals, and harsh environments. Unlike polyamides, phenalkamides are not moisture-sensitive and do not develop blushing when cured in damp or humid areas. They also bond well with a variety of substrates, such as concrete, metal, and other materials, and demonstrate high toughness and flexibility. As a result, they are ideal for use in demanding protective coatings across all-weather environments, combining advantages from both phenalkamines and polyamides.
Considerations and Limitations
Phenalkamides typically have a slightly reduced pot life compared to polyamides but longer than phenalkamines. In extremely cold climates, they cure more slowly than phenalkamines but still faster than polyamides. Like phenalkamines, they may react to moisture during storage and handling before the curing process begins. Therefore, it is essential to store and manage phenalkamide resins in dry environments to prevent moisture uptake or degradation.
Mercaptans
Mercaptans, also referred to as thiol curing agents, are a category of epoxy curing agents that feature sulfur-containing mercaptan or thiol functional groups. These agents react with epoxy resins to create a thioether-based cross-linked network. They provide good flexibility, support curing at low temperatures, and exhibit strong adhesion to various surfaces. They are commonly used in situations where low-temperature or low-viscosity curing is necessary.
Curing Characteristics
Mercaptans support curing processes at low temperatures, including at or below room temperature. This makes them appropriate for systems that require or benefit from low-temperature curing capabilities. They also cure faster than many other agents, including polyamides and anhydrides. This enables quick development of strength and bonding in the final epoxy network. Moreover, mercaptan curing agents generally have low viscosity, improving flow and wetting. These properties make them ideal for uses that demand easy application and low-viscosity formulations.
Performance Properties
Epoxy systems cured with mercaptans demonstrate strong flexibility and toughness. They can endure mechanical loads, impact forces, and vibration, making them ideal for applications that demand durability and flexibility. They also enhance adhesion on various surfaces, such as metal, plastic, and concrete. In addition, mercaptan curing agents usually produce low shrinkage during cure, which helps reduce internal stress in the final product. This feature is valuable in situations where maintaining dimensional accuracy is important.
Considerations and Limitations
The main drawback of mercaptans is their strong, distinct odor, which many users find unpleasant. Proper ventilation and safe handling practices are necessary to reduce exposure to the odor. Some mercaptan-based curing agents may discolor or yellow over time when subjected to sunlight or UV exposure. This could impact the visual appearance of cured products in applications where aesthetics matter. Mercaptan curing agents may react to moisture, so storage and handling must be done in dry environments. Moisture contamination may lead to side reactions or interfere with proper curing of the system.
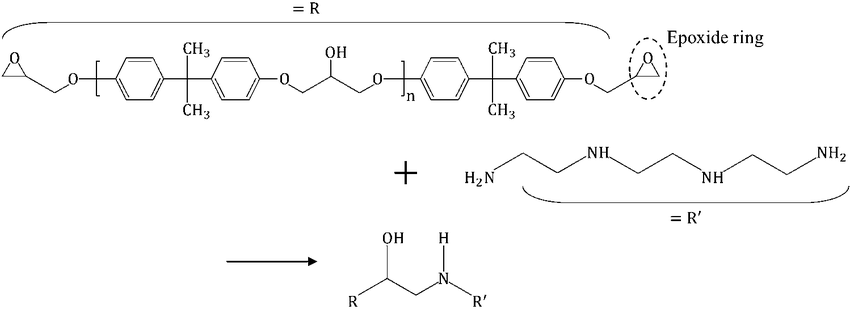
Silane Type Curing Agents
Silane curing agents are derived from silane-based compounds. These molecules are centered around a silicon atom and generally contain two types of functional groups:
- Hydrolyzable Groups:
In this group, R is typically an alkoxy, acyloxy, halogen, or amine group. After hydrolysis, they generate reactive silanol groups that can chemically bond with inorganic surfaces like glass or metal. - Non-Hydrolyzable Reactive Groups:
In this case, X usually represents an amino, epoxy, or vinyl group. These groups form chemical bonds with organic materials such as resin systems, enabling specific performance enhancements.
- Amino-functional silanes serve as conventional amine-type curing agents for epoxy and urethane resins. They promote curing at lower temperatures while improving chemical and corrosion resistance, as well as enhancing adhesion to the substrate.
- Vinyl-functional silanes also help improve bonding to substrates when used as curing agents.
Check out our blog on Coating Dry Film Thickness (DFT) Measurements & Acceptance Criteria – FROSIO
Key Characteristics for Evaluating Curing Agents
Although testing and evaluating a new curing agent can be a detailed process, focusing on critical parameters can help save time and materials while still obtaining meaningful and reliable results.
Before conducting full-scale performance tests, it is essential to determine the correct dosage of the curing agent. While a 1:1 stoichiometric ratio between the curing agent’s reactive groups and the binder’s functional groups is typically a reasonable starting point, desired properties may only emerge when the ratio is adjusted.
For example, an under-cured system (off-ratio) may yield a more flexible film, whereas an over-cured system may enhance chemical resistance.
Moisture control is also highly important, as unwanted moisture can cause side reactions with the curing agent, leading to inconsistent or negative results.
Additional Properties to Evaluate:
- Viscosity: In 1K systems, viscosity helps assess stability, while in 2K systems it is critical for determining pot life. It must be monitored over time and under specific temperatures. Even in 1K systems, curing agents may exhibit latent reactivity depending on the formulation.
- Gloss: Since the curing agent is involved in the film formation process, it can influence the surface gloss of the final film.
- Hardness: The final film’s hardness is also impacted by the curing agent due to its role in network formation.
- Impact Resistance: Film flexibility, as measured by impact tests, can be affected depending on the curing agent used.
- Chemical Resistance: Evaluate resistance to various chemicals such as solvents, acids, alkalis, and water.
- Color Stability and Blushing: Curing agents may cause yellowing over time, especially in heat-cured (stoving) systems. Amine-based curing agents may also lead to blushing when exposed to moisture.
- Overall Appearance: General film quality, uniformity, and surface finish must also be assessed.
Factors Influencing Curing Performance:
- Functionality of the curing agent
- Functionality of the binder
- Dosage and chemical type of the curing agent
- Curing temperature and duration
- Environmental moisture levels
Conclusion
The choice of a curing agent directly impacts the mechanical, thermal, and chemical properties of a coating system. From fast-reacting mercaptans and moisture-tolerant phenalkamines to high temperature resistant anhydrides and versatile polyamides, each curing agent offers distinct benefits tailored to specific end-use requirements.
Evaluating factors such as pot life, curing temperature, chemical compatibility, and environmental conditions helps formulators select the most suitable crosslinker. Moreover, performance testing, covering properties like viscosity, gloss, hardness, and chemical resistance ensures that the final film meets the application’s functional and aesthetic standards. As coating technologies continue to evolve, a well-informed approach to curing agent selection remains key to achieving superior results in both industrial and consumer applications.
Blog Prepared by:
R. Venkatesan
Protective Coating Specialist
Senior Training Instructor for FROSIO / ICorr
+91-9176618930 / info@htscoatings.in / info@frosiotraining.com