Introduction
Epoxy coatings are foundational to modern industrial protection systems. Known for their outstanding adhesion, chemical resistance, and mechanical strength, these coatings are extensively used in industries such as oil & gas, marine, automotive, flooring, and structural steel. However, the effectiveness of an epoxy system depends heavily on two factors: the type of epoxy resin and the curing agent selected.
Understanding these components is critical for engineers, coating professionals, and industrial buyers aiming to optimize performance and service life of epoxy coatings. In this article let’s dive deep into the most common types of epoxy coatings, curing agents, and the best practices for selecting the right epoxy solution.
What Are Epoxy Coatings?
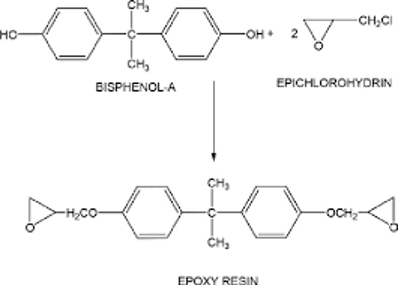
Epoxy resins are thermosetting polymers formed by the reaction of epichlorohydrin with bisphenol-A (or other polyols). These resins contain reactive epoxide groups that allow cross-linking when mixed with suitable curing agents.
Epoxy coatings offer strong bonding to a variety of substrates, including concrete, steel, and composites. Their resistance to chemicals such as oils, solvents, acids, and alkalis makes them a preferred choice for harsh environments. They also exhibit high tensile and compressive strength, contributing to excellent mechanical performance. Furthermore, epoxy coatings are thermally stable and can be formulated to handle elevated service temperatures.
Now let’s look into the different types of epoxy coatings.
Classification of Epoxy Coatings
Solvent-Based Epoxy Coatings
Solvent-based epoxy coatings are traditional formulations in which organic solvents are used to reduce the viscosity of the epoxy resin and facilitate easier application. These systems are known for their excellent penetration characteristics, particularly on porous surfaces such as aged concrete or rusted steel. Once applied, the solvents evaporate, leaving behind a hard, chemically resistant film.
However, the use of these organic solvents contributes to high levels of volatile organic compounds (VOCs), raising concerns regarding worker safety, fire hazards, and environmental impact. Despite these drawbacks, solvent-based epoxies remain widely used in industrial settings where stringent performance standards outweigh environmental restrictions. Common applications include structural steel protection, pipeline coatings, and machinery exteriors.
Water-Based Epoxy Coatings
Water-based epoxy coatings offer an environmentally conscious alternative to solvent-based systems. In these formulations, water serves as the primary diluent, significantly reducing VOC emissions. These coatings are favored for their low odor, ease of cleanup, and safer working conditions.
While they generally do not match solvent-based systems in terms of chemical resistance and film hardness, advances in resin technology have improved their durability and performance. Water-based epoxies cure by coalescence and chemical reaction, often requiring longer drying and curing times. They are particularly suited for indoor applications such as cleanrooms, commercial interiors, food and beverage processing facilities, and pharmaceutical manufacturing spaces.
100% Solids Epoxy
Unlike solvent-based and water-based coatings, 100% solids epoxy coatings do not contain any volatile carriers. This means the entire applied material remains on the surface, allowing the creation of very thick films in a single application. These systems are particularly beneficial in environments where high build and excellent chemical resistance are necessary.
Due to the absence of solvents, they offer zero VOC emissions, making them compliant with strict environmental regulations. However, the application requires specialized techniques due to short pot life and high viscosity. These coatings are commonly used in industrial flooring, containment linings, and tank interiors, where their resistance to abrasion and chemicals is critical.
Powder Epoxy Coatings
Powder epoxy coatings are dry, thermosetting materials applied electrostatically to a substrate and cured under heat. During the curing process, the powder melts, flows, and chemically reacts to form a hard, durable coating.
This type of coating offers several advantages, including a uniform finish, minimal waste, and no VOC emissions. It provides excellent mechanical strength and abrasion resistance, making it suitable for parts subjected to physical stress. However, powder coatings require curing ovens, limiting their use to factory-based operations. Typical applications include automotive components, appliances, and reinforcing bars (rebar).
Fusion-Bonded Epoxy (FBE)
Fusion-bonded epoxy is a specialized type of powder coating applied to heated metal surfaces, typically by electrostatic spray. The heat melts and fuses the epoxy particles into a continuous, strongly adherent film. Once cured, FBE provides a seamless, corrosion-resistant barrier.
FBE coatings are widely used in pipeline protection and rebar for concrete reinforcement. Their ability to form a chemically resistant interface with steel makes them ideal for oil and gas pipelines, offshore structures, and other applications exposed to harsh environments. Despite the need for high-temperature curing and complex equipment, the long-term performance of FBE coatings justifies their use in infrastructure projects.
High-Build Epoxies
High-build epoxy coatings are designed to be applied in thick layers, typically exceeding 300 microns in a single coat. These systems are formulated with thixotropic agents that prevent sagging during application and promote uniform film thickness.
High-build formulations are essential in situations where long-term protection is required without the time and cost of multiple coat applications. They are frequently used in marine environments, structural steel, tank exteriors, and bridges, where higher resistance to moisture, salt spray, and mechanical damage is needed.
Specialty Formulations
Specialty epoxy formulations, such as novolac epoxies, are engineered for specific high-performance requirements. Novolac resins, derived from phenol and formaldehyde, provide exceptional chemical and heat resistance. These systems can withstand exposure to strong acids, solvents, and elevated temperatures up to 200°C.
Due to their superior resistance characteristics, novolac epoxies are used in the most demanding industrial environments, including chemical processing plants, acid storage tanks, and high-temperature pipelines. Although they tend to be more expensive and complex to apply, they are very reliable to be used in high risk applications.
What are Curing Agents?
A curing agent (also called a hardener) in epoxy resins is a chemical compound that reacts with the epoxy resin (typically a diglycidyl ether of bisphenol A or similar epoxide) to initiate the curing process, which transforms the liquid resin into a solid, thermoset polymer.
The curing agent, or hardener, initiates cross-linking of epoxy resins, forming a rigid, thermoset network. This curing process defines the mechanical strength, chemical resistance, and heat tolerance of the final coating. We have various classes of curing agents, each tailored to specific performance and application requirements.
What happens step by step during curing:
- Mixing Stage: The epoxy resin (with reactive epoxide groups) is mixed with the curing agent. The most widely used epoxy resin is bisphenol-A diglycidyl ether (DGEBA), which has two terminal epoxide groups capable of undergoing nucleophilic ring-opening reactions:
Structure of DGEBA:
CH₂–CH–CH₂–O–(CH₃)₂C₆H₂–C₆H₂(CH₃)₂–O–CH₂–CH–CH₂
| |
O O
Each epoxide ring is a strained three-membered cyclic ether, highly electrophilic and prone to nucleophilic attack, especially under the influence of amines or other curing agents.
- Chemical Reaction Begins: The curing agent contains active hydrogen atoms or other reactive groups (like amines, anhydrides, or acids) that open the epoxide rings.
- Crosslinking Occurs: Epoxy resins undergo step-growth polymerization through a chemical reaction between epoxide groups and active hydrogen-containing curing agents, forming a thermoset network. The curing (or cross-linking) process is irreversible and results in a rigid, three-dimensional polymer matrix with high mechanical strength, chemical resistance, and thermal stability.
The final cross-link density governs the mechanical and thermal properties of the cured epoxy. Higher cross-linking leads to:
- Increased glass transition temperature (Tg)
- Improved solvent and chemical resistance
- Higher tensile and flexural strength
- Reduced elongation and toughness
Glass Transition Temperature (Tg) is a critical performance indicator and is typically:
- 50–80°C for polyamide-cured systems
- 90–150°C for cycloaliphatic amines
- 180°C for novolac–anhydride systems
Physical Changes During Cure
The physical transformation from liquid to solid passes through three key stages:
- Liquid (Low viscosity) – excellent substrate wetting.
- Gelation (Sol-Gel transition) – onset of cross-linking and loss of flow.
- Solid Thermoset – network rigidity and final properties develop.
Differential Scanning Calorimetry (DSC) is commonly used to monitor cure progression via exothermic heat flow measurements.
Key Parameters Influencing Curing:
- Stoichiometric Ratio of resin to hardener (ideally near 1:1 equivalent ratio)
- Cure Temperature and Time
- Presence of Catalysts
- Moisture and Impurities
Two primary classes of curing agents dominate industrial use: amines (primary, secondary, or tertiary) and anhydrides (cyclic and aliphatic).
Amine Curing Agents
Amine curing agents are the most widely used in industrial epoxy systems due to their versatile performance profiles. They contain active hydrogen atoms (–NH₂, –NH–) that react with the epoxide groups in the resin, initiating the crosslinking (curing) process.
Amine-Based Curing Mechanism
Amine curing involves nucleophilic attack by primary (R–NH₂) or secondary (R₂–NH) amines on the electrophilic carbon of the epoxide group. The basic mechanism includes two steps:
(a) Primary Amine Reaction:
A primary amine opens the epoxide ring to yield a secondary amine and a hydroxyl group:
Reaction:
R–NH₂ + R’–CH–CH₂–O → R–NH–CH₂–CH(OH)–R’
(b) Secondary Amine Reaction:
The newly formed secondary amine can further react with another epoxide to form a tertiary amine:
Reaction:
R–NH–CH₂–CH(OH)–R’ + R”–CH–CH₂–O → R–N(CH₂–CH(OH)–R’)(CH₂–CH(OH)–R”)
This step-growth process continues until the resin becomes a highly cross-linked network. Each amine hydrogen contributes to a cross-link, and the resulting hydroxyl groups can form hydrogen bonds, increasing cohesion and polarity.
Reaction Scheme Summary:
R–NH₂ + Epoxide → R–NH–CH₂–CH(OH)–R’
R–NH– + Epoxide → R–N(CH₂–CH(OH))₂
Aliphatic Amines
These are straight-chain or branched amines offering rapid reactivity at ambient temperatures. They deliver strong chemical resistance and good adhesion but may suffer from amine blush when exposed to moisture during curing. Aliphatic systems are suitable for general-purpose coatings, maintenance applications, and protective primers.
Cycloaliphatic Amines
Cycloaliphatic amines combine the reactivity of aliphatic amines with improved UV and chemical resistance. Their ring structures offer better mechanical strength and reduced yellowing. Cycloaliphatics are frequently used in high-performance coatings where appearance and durability are critical, such as in flooring or exterior steel structures.
Aromatic Amines
These are based on benzene ring structures and are typically used in heat-cured systems. Aromatic amines offer excellent chemical and thermal resistance but are generally slower to react. Their use is declining due to toxicity and handling concerns, though they remain relevant in heavy-duty industrial linings.
Anhydride Curing Agents
Anhydrides are cyclic compounds that react slowly with epoxy groups and typically require elevated temperatures to cure. They offer low exothermic reactions, making them ideal for thick film or cast applications. Anhydride systems provide exceptional resistance to solvents, acids, and heat, and are often used in chemical process vessels, pipelines, and electrical insulation applications.
Anhydride-Based Curing Mechanism
Anhydrides cure epoxy resins via ring-opening esterification reactions. This process requires elevated temperatures and typically proceeds through a two-step mechanism:
(a) Initiation by Hydroxyl Groups:
A hydroxyl group, either present in the resin or introduced during processing, opens the anhydride ring to form a monoester:
Reaction:
R–CO–O–CO–R’ + HO–CH₂–CH– → R–COO–CH₂–CH(OH)– + R’–COOH
(b) Propagation by Carboxylic Acid:
The carboxylic acid formed then reacts with an epoxy group to yield a second ester linkage:
Reaction:
R’–COOH + CH₂–CH–O → R’–COO–CH₂–CH(OH)
The overall process leads to linear and cross-linked ester networks with very high thermal and chemical resistance.
Polyamide & Polyamine Blends
Polyamides are derived from fatty acids and provide flexibility, good adhesion, and tolerance to humid surfaces. They are commonly blended with polyamines to create formulations that strike a balance between flexibility and hardness. These curing systems are widely used in marine and maintenance coatings due to their durability and surface-tolerant properties.
Phenalkamines and Phenolic Hardeners
Phenalkamines are derived from natural oils like cardanol and exhibit fast reactivity even at low temperatures. Their moisture tolerance and rapid cure make them suitable for cold-weather applications. Phenolic hardeners, often used in combination with novolac epoxies, provide enhanced chemical and thermal resistance. They are typically used in linings and secondary containment systems.
Curing Agent Type | Pot Life | Chemical Resistance | Cure Speed | Temp. Resistance |
Aliphatic Amine | Short | Moderate | Fast | Moderate |
Cycloaliphatic Amine | Medium | High | Medium | High |
Aromatic Amine | Long | Very High | Slow | Very High |
Anhydride | Very Long | Excellent | Very Slow | Excellent |
Polyamide | Medium | Moderate | Medium | Moderate |
Phenalkamine | Short | Moderate | Very Fast | Moderate |
Amine vs Anhydride Systems: Technical Comparison
Cure Mechanisms
Amine-cured epoxy systems rely on the nucleophilic attack of amine groups on the epoxide rings, forming a tight, cross-linked network. This process can occur at ambient temperatures (particularly for aliphatic and cycloaliphatic amines), making them suitable for field applications.
Anhydride curing involves ring-opening reactions with the epoxy groups, typically requiring elevated temperatures (>80°C). The slower reaction rate and low exotherm make anhydrides ideal for thick-section castings or high-solids linings where thermal control is essential.
Thermal and Chemical Resistance
Amine-cured systems, especially aromatic types offer excellent thermal stability and resistance to aggressive solvents and acids. However, their performance can vary significantly depending on humidity and formulation.
Anhydride-cured epoxies consistently exhibit superior resistance to acids, alkalis, and solvents, along with excellent dielectric properties. Their high-temperature resistance surpasses most amine-based systems, especially in heat-cured novolac-anhydride systems used for chemical storage or process tank linings.
Industrial Relevance and Performance Characteristics
Amine systems dominate general industrial applications due to their versatility and ease of use at room temperature. Their rapid cure and mechanical robustness make them ideal for coatings in marine, flooring, and structural steel sectors.
Anhydride systems, while requiring more sophisticated application and curing conditions, are the go-to choice in environments demanding chemical resilience and dimensional stability such as chemical processing plants, semiconductor equipment, and high-voltage electrical insulation.

Selection Criteria for Epoxy Coatings
Substrate Type
Concrete substrates benefit from 100% solids or water-based epoxy formulations that offer high adhesion and penetration. Steel structures are better served with solvent-based epoxies or FBE systems that provide robust corrosion protection. For composite or mixed substrates, compatibility testing is essential to ensure adhesion and cure reliability.
Application Conditions
Environmental factors play a crucial role in the success of an epoxy application. In high-humidity or cold-temperature environments, phenalkamines or polyamides are preferred due to their tolerance to moisture and ability to cure under less-than-ideal conditions. For applications in controlled environments, such as OEM facilities, more complex systems like anhydrides can be employed effectively with heat curing.
Mechanical Load and Chemical Exposure
Heavy mechanical stress or abrasive environments require high-build, novolac, or aromatic amine-cured systems that can endure wear and maintain integrity. In areas with exposure to harsh chemicals, including acids, solvents, or hydrocarbons, anhydride-cured or novolac epoxies are recommended for their superior chemical resistance.
VOC Regulations
Environmental compliance is increasingly influencing material selection. Water-based and 100% solids epoxy systems are preferred in regions with strict VOC restrictions, offering performance without compromising safety or emissions compliance. Powder coatings, such as FBE, offer a VOC-free solution when suitable application equipment is available.
Safety Considerations
Worker safety and operational risk must also be factored into system selection. Solvent-based systems pose flammability and inhalation hazards, necessitating adequate ventilation and PPE. Conversely, water-based and phenalkamine systems offer lower toxicity profiles and are more forgiving in field applications. Proper handling protocols must always be followed based on the curing agent and application conditions.
Applications of Epoxy Coatings
Let’s have a look at the industrial applications of epoxy coatings.
Marine and Offshore Environments
Epoxy coatings are indispensable in marine and offshore industries where structures are constantly exposed to saltwater, high humidity, and mechanical stress. High-build epoxy primers and barrier coats, often cured with moisture-tolerant polyamides or phenalkamines, are used to protect ship hulls, decks, and ballast tanks. These systems provide exceptional corrosion resistance even under immersed conditions.
In offshore platforms and subsea structures, epoxy coatings, particularly novolac-based systems, serve as internal linings for tanks and pipes that handle aggressive seawater or chemically treated ballast water.
Electrical and Electronics Applications
Epoxy resins are used in the electronics industry not only as protective coatings but also as encapsulants and adhesives. Anhydride-cured epoxy systems, known for their excellent dielectric properties and thermal stability, are employed in the potting of circuit boards, transformers, and electrical connectors. These systems minimize moisture ingress and improve electrical insulation.
Cycloaliphatic epoxy systems are preferred in high-voltage applications where UV resistance and thermal endurance are essential. The low shrinkage and high dimensional stability of cured epoxies are critical for maintaining performance in miniaturized electronic assemblies.
Industrial Flooring and Infrastructure
Epoxy flooring systems are widely used in industrial and commercial buildings where resistance to abrasion, chemicals, and heavy mechanical loads is required. 100% solids epoxy coatings are favored for their ability to form seamless, high-build surfaces that are impervious to moisture and easy to clean. These systems are commonly used in pharmaceutical plants, food processing areas, hospitals, and warehouses. The addition of quartz aggregates, colored flakes, or anti-slip agents can enhance both performance and appearance.
Oil, Gas, and Chemical Processing Facilities
Epoxy coatings play a critical role in the oil, gas, and chemical industries, where infrastructure is subjected to highly corrosive environments. Novolac epoxy systems are often selected for their superior chemical resistance and high-temperature performance. These coatings are used to line storage tanks, pressure vessels, separators, and chemical reactors that are exposed to acids, solvents, and hydrocarbons. Anhydride-cured epoxy systems are commonly employed for thick-film linings in containment areas and tank internals, offering excellent resistance to immersion and thermal cycling. Fusion-bonded epoxy (FBE) coatings are applied to pipelines to prevent corrosion and cathodic disbondment, significantly extending asset service life in harsh field conditions.
Water and Wastewater Treatment Plants
In municipal and industrial water treatment plants, epoxy coatings are essential for protecting concrete and steel structures from chemical attack and water ingress. Solvent-free epoxy systems are used to line clarifiers, aeration tanks, and digesters, forming impermeable membranes that resist chemical erosion and microbiologically induced corrosion (MIC). Moisture-tolerant polyamide systems allow application on damp substrates, which is often necessary in field repair conditions. These coatings ensure long-term protection of infrastructure components that operate in aggressive aqueous environments with variable pH and contaminant load.
Also read – Coating Curing Agents and Formula – FROSIO
Civil Engineering and Structural Steel Protection
Structural steel elements in bridges, high-rise buildings, stadiums, and industrial plants are often coated with epoxy-based primers and intermediate coats. Zinc-rich epoxy primers are used as the first line of defense against corrosion, providing sacrificial protection and promoting adhesion for subsequent layers. Micaceous iron oxide (MIO)-filled high-build epoxy systems are frequently applied as barrier coats to enhance impermeability and durability. For reinforced concrete applications, fusion-bonded epoxy coatings are applied to rebar to prevent chloride-induced corrosion and maintain bond integrity within the concrete matrix. These coatings are compatible with polyurethane or polysiloxane topcoats used in long-life coating systems.
Conclusion
Epoxy coatings remain a crucial component of protective and functional surface engineering across a wide range of industrial applications. Their success lies in the versatility of epoxy resin chemistries and the diverse range of curing agents that enable fine-tuning of performance characteristics such as adhesion, chemical resistance, thermal stability, and mechanical strength. Whether selecting between solvent-based, water-based, 100% solids, or specialty novolac systems, the right formulation must align with the service environment, substrate conditions, and regulatory requirements. As demands for performance, sustainability, and safety continue to grow, epoxy technologies will continue evolving, offering new opportunities for innovation in coatings science and engineering.
Blog Prepared by:
R. Venkatesan
Protective Coating Specialist
Senior Training Instructor for FROSIO / ICorr
+91-9176618930 / info@htscoatings.in / info@frosiotraining.com