Detrimental Effects of Soluble Salts on Coating Systems
Factors Influencing the Rate of Coating Failure
Methods of Detecting Soluble Salts in Coatings
Bresle Method (ISO 8502-6 and ISO 8502-9)
Standards and Acceptable Limits of Salt in Coatings
Best Practices Before Coating Application
Testing Frequency and Statistical Sampling
Coating failures is a critical problem that can affect the safety of equipment and structures. Protective coatings are an important element of corrosion control systems across industries including marine, oil and gas, infrastructure, and petrochemical. However, the effectiveness and durability of these coatings can be severely compromised by the presence of soluble salts on substrate surfaces prior to coating application. Despite their often-invisible presence, soluble salts such as chlorides, sulfates, and nitrates can catalyze underfilm corrosion, leading to premature coating breakdown, blistering, and adhesion loss. In this article we will look into the nature of soluble salts, their formation mechanisms, their detrimental effects on protective coatings, how they lead to coating failures, detection methods, and applicable industry standards.
Want to know more about adhesion between coating and substrate? Check out our blog on COATING’S ADHESION & COHESION
What are Soluble Salts?
Soluble salts are hygroscopic inorganic compounds that can dissolve in water and are commonly found on metallic surfaces exposed to natural or industrial environments. The primary types of soluble salts relevant to corrosion and coating performance are chlorides, sulfates, and nitrates. These salts are commonly deposited onto metallic surfaces through environmental exposure, including airborne sea spray, industrial emissions, deicing salts, and rainwater containing dissolved contaminants.
In marine and coastal environments, for instance, airborne chlorides are a significant concern. Wind-driven salt particles can settle on metallic structures and, in the presence of moisture, dissociate into ionic species that penetrate the metal-coating interface. In industrial zones, SO2 and NOx emissions can interact with moisture to form sulfuric and nitric acids, which further degrade into sulfate and nitrate ions upon contact with surfaces.
Soluble salts can also originate from internal processes such as hydroblasting with impure water or contaminants in storage and processing chemicals. Deicing operations, particularly in transportation infrastructure and airport maintenance, introduce high levels of chloride and nitrate salts to exposed steel. Additionally, groundwater or recycled wash water used during surface preparation may contain residual salts that contribute to the contamination load.
These salts are not always visible to the naked eye and can remain on a substrate even after dry abrasive blasting, which is traditionally considered a standard surface preparation method. Consequently, unremoved soluble salts can initiate localized corrosion under otherwise sound coatings.
These salts are not always visible to the naked eye and can remain on a substrate even after dry abrasive blasting, which is traditionally considered a standard surface preparation method. Consequently, unremoved soluble salts can initiate localized corrosion under coatings.
How do Soluble Salts cause Coating Failures?
Soluble salts pose a considerable risk to the long-term performance of protective coatings due to their ability to attract and retain moisture. When a protective coating (e.g., epoxy, polyurethane) is applied over a substrate contaminated with soluble salts, these salts become entrapped beneath the coating film. If surface preparation is inadequate (poor blasting, insufficient washing, or ineffective soluble salt removal), these contaminants are sealed within the coating-substrate interface. Importantly, salts such as chlorides are deliquescent—they not only attract water vapor but can also dissolve in minute amounts of absorbed moisture, even before visible water droplets form. The applied coating acts as a semi-permeable membrane, unknowingly locking in this hidden source of corrosion potential.
When moisture comes into contact with salt anions, it promotes water polarization, effectively separating into H⁺ and OH⁻ ions. This interaction facilitates the dissolution of the metal surface, leading to the formation of iron chloride—classified as an acid salt. As the anions react with the metal in the presence of moisture trapped beneath the coating, a conductive path is established, accelerating corrosion. In essence, the presence of salt anions enhances metal hydration (dissolution) and increases the metal’s tendency to release ions into solution, thereby promoting metal loss.
Anodic and Cathodic Region Dynamics
Within the metal surface, a conductive pathway allows electron flow from the anodic (acidic) region—where metal dissolution occurs (pitting)—to surrounding cathodic (alkaline) areas. This electron movement balances the localized anionic activity within the pit. As a result, alkaline cathodic sites form around the acidic anodic zones, explaining why pitting corrosion remains highly localized.
At these cathodic sites, chemical reactions such as the combination of sodium ions (from chloride salts) with hydroxide ions (from water) produce sodium hydroxide (NaOH), further increasing local alkalinity. This concentrated alkalinity attracts more water to the cathodic area, establishing an osmotic pressure gradient that sustains the corrosion process.
This can lead to two major coating failure mechanisms: osmotic blistering and underfilm corrosion. Let’s look into each one of this in detail:
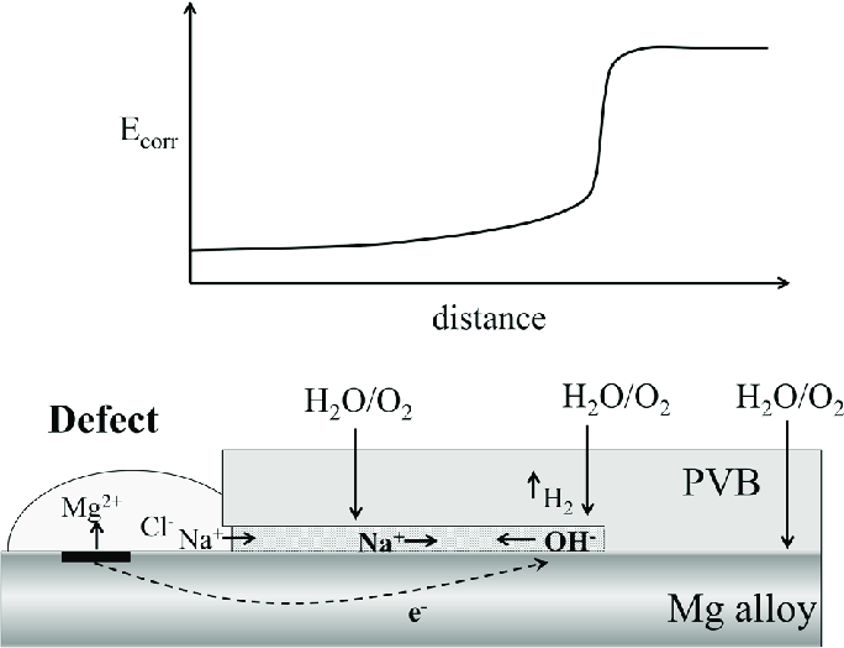
Osmotic Blistering
Osmotic blistering is driven by the differential osmotic pressure created when soluble salts remain on the substrate under a coating. Once a concentrated salt solution forms beneath the coating, a significant osmotic pressure differential arises between this solution and the relatively dilute moisture outside the coating. The coating, acting as a semi-permeable membrane, allows water molecules to pass through but restricts the movement of ions.
To equilibrate this osmotic gradient, water from the external environment is continuously drawn through the coating into the salt-laden pocket. This influx of water increases the internal volume of the salt solution, exerting mechanical stress on the coating. Over time, this results in the formation of blisters localized swellings or domes in the coating surface known as osmotic blistering. These blisters are often filled with a corrosive brine and represent areas of extreme mechanical and chemical degradation.

Underfilm Corrosion
Underfilm Corrosion is a common form of coating failure. The concentrated salt solution beneath the blister is highly conductive and sets up an electrochemical cell at the metal interface. The anodic sites on the metal surface begin to oxidize, releasing metal ions (e.g., Fe²⁺ for steel), while cathodic reactions, typically oxygen reduction, occur nearby. This leads to the formation of corrosion products such as iron oxides and hydroxides. Unlike uniform corrosion, this underfilm corrosion is localized and aggressive, fueled by the trapped electrolyte and continuous oxygen diffusion through microdefects in the coating.
As corrosion products accumulate beneath the coating, they exert additional mechanical stresses, causing localized delamination or disbondment of the coating from the substrate. The volume expansion associated with corrosion products (e.g., iron oxides having larger volume than metallic iron) further lifts the coating, exposing fresh substrate to corrosive attack. This creates a self-sustaining cycle where more salts get activated, more moisture is drawn in, and corrosion accelerates. The once-protective coating now becomes a trap for moisture and contaminants, allowing underfilm corrosion to spread radially from the initial defect or salt pocket.
If unchecked, osmotic blistering and underfilm corrosion will eventually lead to widespread coating failure. Large areas of the coating may disbond, exposing bare metal to direct environmental attack. The visual appearance of blistering, rust staining, and flaking paint is merely the surface manifestation of a much deeper and more damaging sub-coating corrosion process. Structural integrity, aesthetic quality, and functional performance of the coated asset are compromised, necessitating costly repairs or replacements.
Factors Influencing the Rate of Coating Failures
Accurately estimating how much soluble salts shorten a coating’s service life is challenging. The detrimental effects of soluble salt contamination do not diminish over time. Under certain conditions, coatings may fail within a year, whereas in other scenarios, similar failures could take several years to develop. Several environmental and material-related factors govern how quickly coatings deteriorate due to soluble salts:
- Nature and Concentration of Contaminants
- Higher salt concentrations accelerate corrosion and coating degradation.
- Chloride-based salts pose a greater risk compared to sulfate salts.
- Coating Type and Film Thickness
- Zinc-rich primers generally exhibit better resistance to salt contamination.
- Coatings with lower water permeability degrade slower than those with higher permeability.
- Thicker coatings provide a more effective barrier, slowing down water ingress compared to thinner applications of the same material.
- Temperature Influence
- Elevated temperatures increase water permeability through coatings and accelerate corrosion rates.
- Exposure Conditions
- Coating systems in atmospheric exposure tolerate soluble salts better than those used in immersion services.
- Laboratory studies indicate that linings in gasoline-water immersion environments are more susceptible to chloride-induced failure than those in water-only immersion.
Want to know more about Protective Coatings? Check out our post – Protective Coatings

Methods of Detecting Soluble Salts in Coatings
Given their potential to remain undetected visually, reliable methods are essential for quantifying the presence of soluble salts before coating application. Several standardized test methods have been developed to evaluate surface cleanliness, with a focus on chloride contamination due to its prevalent role in corrosion.
Bresle Method (ISO 8502-6 and ISO 8502-9)
The Bresle method is among the most widely used techniques for field detection of soluble salts. The Bresle method quantifies the total concentration of soluble salts (as conductivity in µS/cm or mg/m²) on a prepared surface to determine whether it is clean enough for coating application.
It is standardized by:
- ISO 8502-6: Extraction of soluble contaminant
- ISO 8502-9: Conductivity measurement and evaluation
The Bresle test works on the principle of extracting soluble salts from the surface into a known volume of deionized water enclosed in a sealed patch, and then measuring the electrical conductivity of the resulting solution. Soluble salts ionize in water, increasing its electrical conductivity. Hence, the higher the conductivity of the extract, the higher the salt contamination on the metal surface.
The metal surface is cleaned to remove dust or loose particles but must not be treated with water, as it could remove the salts before testing. The test patch must be placed on a representative area that is suspected to have salt contamination. The adhesive Bresle patch is firmly pressed onto the surface to form a watertight seal. Air bubbles are removed to prevent leakage or false readings. Using a syringe, a known volume (usually 3.0 mL) of deionized water is injected into the patch cavity through the septum. The solution inside the patch is manually agitated for about 2–3 minutes by gently massaging the patch. This allows the salts to dissolve into the water. The solution is then carefully withdrawn with the same syringe and transferred into a clean beaker or test tube. Using a calibrated conductivity meter, the conductivity of the withdrawn solution is measured in microSiemens per centimeter (µS/cm).
Chloride Test Kits
Chloride-specific test kits based on ion-selective electrodes or titration methods offer another avenue for assessing contamination. These kits can detect chloride levels down to a few mg/m2, providing more targeted information than conductivity-based tests.
The most common chloride test kits in use today operate on a colorimetric tube method, which is the basis of ISO 8502-5. In this method, salts are first extracted from a known surface area—typically 10 cm²—using deionized water or a proprietary extraction solution held within an adhesive sleeve or patch. The solution dissolves soluble salts from the surface. This extracted liquid is then introduced into a specially treated glass titration tube containing silver nitrate and chromate indicator reagents. Chloride ions react with silver to form silver chloride, which precipitates as a white deposit. As the chloride ions are consumed, the chromate indicator shifts color, forming a clear boundary line from white to pink within the tube. The position of this boundary corresponds to a calibrated chloride concentration, allowing the user to read the result directly in micrograms per square centimeter (µg/cm²).
Typically, these kits can detect chloride levels ranging from 1 to 60 µg/cm², which is sufficient for most industrial surface preparation standards. The testing process is rapid, providing results in under two minutes, making them suitable for field inspections and on-site quality control.
The primary advantage of these kits is their high specificity to chloride ions. They are unaffected by other soluble salts like sulfates or nitrates, which can interfere with non-specific methods such as conductivity measurements. Additionally, they do not require any electronic equipment, power sources, or complex calibration procedures, making them ideal for use in challenging field conditions.
However, these kits do have limitations. Each test requires disposable components—namely the adhesive sleeve and titration tube—which can become costly for large-scale testing. Moreover, these kits measure only chloride ions. If other contaminants like sulfates or nitrates are present and of concern, separate tests or more comprehensive CSN (Chloride, Sulfate, Nitrate) kits must be used. Another limitation is that very high salt loads can exceed the measurement range of the titration tube, necessitating dilution or alternative methods like conductivity testing.

Surface Sampling Methods
Surface sampling methods are techniques used to extract and analyze contaminants such as soluble salts, dust, oil, and other residues from steel surfaces to assess surface cleanliness prior to coating. These methods are critical because invisible contaminants, especially chlorides, sulfates, and nitrates, can severely compromise coating adhesion, leading to premature failure through mechanisms like osmotic blistering and underfilm corrosion. The selection of a sampling method depends on the type of contaminant being tested, the required sensitivity, and the testing environment (field or lab).
For ion-specific detection, such as measuring chloride contamination, the ISO 8502-5 method involves using adhesive sleeves or patches for salt extraction, followed by analysis with chloride detection tubes. These tubes contain reagents that react with chloride ions, causing a color change that is proportional to chloride concentration. This technique is precise and widely used for field testing because it directly quantifies chloride without interference from other salts.
Another surface sampling method involves swabbing or wiping, where a clean cloth, sponge, or filter paper moistened with deionized water is used to physically wipe a known area of thesurface. The swab is then rinsed in water, and the resulting solution analyzed using titration, ion-selective electrodes, or laboratory-based ion chromatography. This method is simple and useful for irregular or large surfaces but may be less accurate in quantifying exact contamination levels compared to sealed extraction techniques.
Advanced methods include pressurized rinsing (ISO 8502-4), where deionized water is sprayed onto the surface under controlled pressure to wash off contaminants. The runoff is collected and analyzed for salts, often in laboratory settings. This method is suitable for large structures like ship hulls or pipelines where patch methods are impractical.
In specialized cases, acid extraction methods (ISO 8502-15) are used to dislodge tightly bound salts or those trapped in mill scale or rust layers. This involves applying a dilute acid solution to the surface, which dissolves embedded salts more effectively than water alone. The extract is then analyzed for specific ions.
Standards and Acceptable Limits of Salt in Coatings
Multiple international standards provide guidance on the acceptable levels of soluble salts before coating application. These include:
- ISO 8502 Series: Particularly Parts 6 and 9, which define the Bresle method for extracting and measuring soluble salts.
- SSPC-SP COM (Soluble Salt Criteria): Offers project-specific criteria for acceptable salt levels depending on the coating system and service environment.
- NACE SP0508-2008: A standard practice that outlines procedures for controlling soluble salt contamination.
ISO 8502 Series – International Foundation for Surface Cleanliness
The ISO 8502 suite provides the most widely recognized and globally accepted framework for soluble salt detection. ISO 8502-6 defines the procedures for extracting salts using the Bresle patch, while ISO 8502-9 specifies the conductometric method for quantifying total salt concentration.
These standards do not impose a universal limit but rather serve as test protocols. However, ISO technical papers and coatings manufacturers often suggest acceptable surface salt levels of:
- ≤ 20 mg/m² for general atmospheric exposure
- ≤ 10 mg/m² for immersion service
- ≤ 5 mg/m² for highly aggressive environments, such as offshore splash zones or chemical plants
It is worth noting that ISO emphasizes that these thresholds should be verified against the performance requirements of the specific coating system and project conditions.
Best Practices Before Coating Application to avoid Coating Failures
Based on industry experience and empirical data, the following best practices are recommended:
- Conduct a risk assessment based on environmental exposure, substrate condition, and coating requirements.
- Always test for soluble salts after surface preparation, particularly after abrasive blasting.
- Use the Bresle method or equivalent testing for field quantification.
- Ensure surface salt levels are within acceptable thresholds before proceeding with primer or coating application.
- Where necessary, employ salt removal techniques such as high-pressure water jetting (≥20,000 psi), steam cleaning, or use of surface-active chemical treatments specifically designed to solubilize and remove salts.
Testing Frequency and Statistical Sampling to avoid Coating Failures
Large surfaces cannot be economically tested in entirety. Therefore, industry practice—based on SSPC and NACE recommendations—suggests:
- One Bresle test per 5–10 m² for general industrial work
- Higher frequency in areas subject to high chloride exposure (e.g., splash zones, joints, or crevices)
- Statistical sampling plans for large projects, where testing locations are determined by grid systems or risk-based approaches
Common Removal Methods for Soluble Salts
Effectively removing soluble salts requires procedures beyond conventional dry abrasive blasting. Three main categories of removal methods are commonly employed: high-pressure water jetting, post-blast washing, and chemical treatments.
High-pressure water jetting (typically 10,000 to 40,000 psi) effectively dislodges and dissolves salts adhered to the surface and within surface profile valleys. Ultra-high pressure (UHP) water jetting above 30,000 psi can also be employed in conjunction with rotating nozzles for uniform cleaning. However, the use of water requires careful control of flash rusting, particularly in humid environments, which can be managed through the use of corrosion inhibitors or dehumidification.
Post-wash rinsing after dry abrasive blasting is another common method. It involves thorough washing of the blasted surface using clean potable or deionized water to remove salts embedded in the profile. This step is sometimes overlooked but can significantly reduce residual contamination.
Chemical treatments such as ammonium bifluoride or surfactant-based salt removers are specifically formulated to bind and solubilize salts on steel surfaces. These chemicals are applied, allowed to dwell, and then rinsed off, typically in controlled shop environments. While effective, these methods require compatibility verification with the subsequent coating system.
Check out our blog on Coating Dry Film Thickness (DFT) Measurements & Acceptance Criteria – FROSIO
Conclusion
Soluble salts, though often invisible, leads to unprecedented coating failures and affects the performance and longevity of protective coating systems. Their presence can trigger deleterious effects like osmotic blistering and underfilm corrosion, both of which undermine the functional integrity of coatings. Detection through standardized methods such as the Bresle test, adherence to industry-defined acceptable limits, implementation of comprehensive surface preparation protocols, and use of effective removal techniques are essential to mitigate these risks. For corrosion engineers and coating professionals, understanding and managing soluble salts is not a secondary concern but a frontline defense in extending asset life and ensuring coating reliability.
Blog Prepared by:
R. Venkatesan
Protective Coating Specialist
Senior Instructor / Trainer for FROSIO / ICORR
+91-9176618930 / info@htscoatings.in / info@frosiotraining.com





One Response